Product
6 min read
FDA Compliance: Why Paper-Based Documents Won't Work for Long

Modern documentation practices are becoming increasingly essential to achieving FDA compliance with the recent updates in the FDA’s approach to safety. While the importance of product quality and safety isn’t news, updated documentation standards outlined in the FSMA and the CGMP are.
A New Era in Food & Beverage Manufacturing
The Food Safety and Modernization Act (FSMA) was signed into law in 2011 with the goal of improving preventative food sanitation and safety measures. This shift, from reactive to proactive, includes some of the biggest changes to food production regulation in over 70 years.
The underlying solution for the FDA’s multitude of proactive strategies is, guess what? Good documentation.
We’ve spent over a decade developing a digital documentation solution for manufacturers, and have worked with countless food & beverage companies to understand industry challenges.
"Documentation will likely be the most substantial
change — and challenge — for manufacturers [...]
they need to back up all of their assessments,
testing and plans with meticulous records."
— Carolyn Heneghan, FoodDive
Reliable Safety Training
Standardization and data collection are critical to safety and efficiency. Manufacturers are not only required to meet FDA regulatory guidelines, but tracking safety information is paramount to ensure that your products are safe for consumption.
Standardized and verified training safety measures decreases the probability that workers will make costly—or dangerous—mistakes. The Current Good Manufacturing Practices (CGMP) outlines two key conditions for this increased reliability on proper training.
FDA Requirement
"Training must be delivered in a manner that can be easily understood by the worker."
(Food CGMP Modernization, Training)
The Dozuki Solution: Improved Training Comprehension
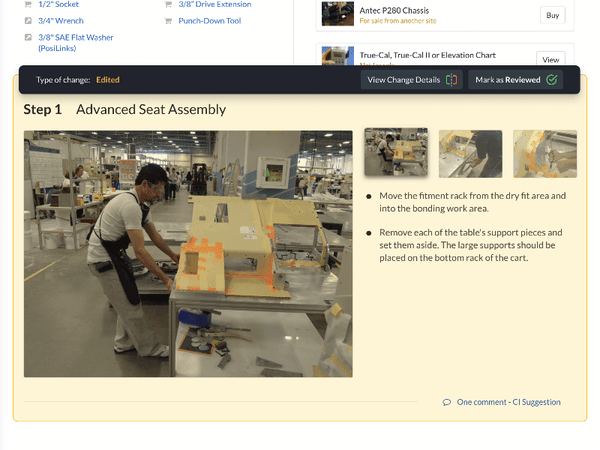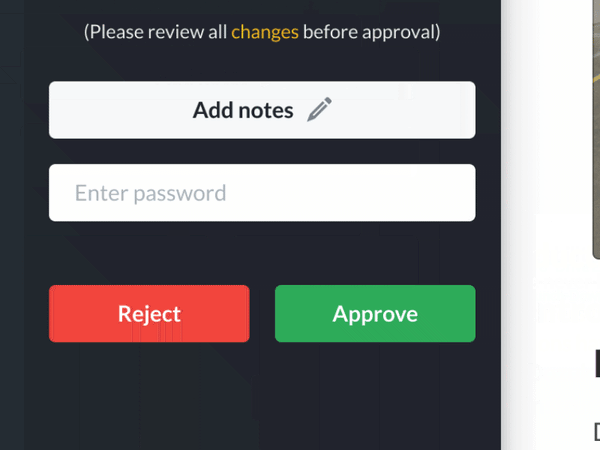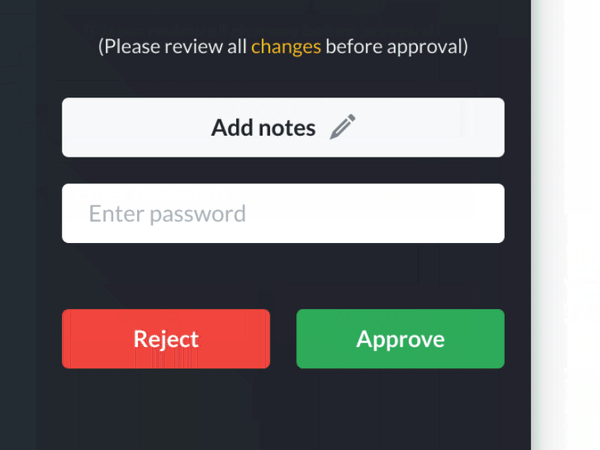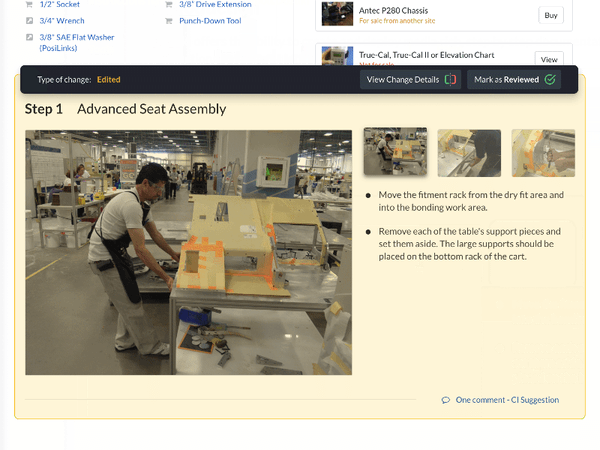
Text-heavy documents with convoluted steps and unnecessary industry jargon make for poor training materials. Standard work instructions in Dozuki supplement text-heavy documents with clear images to help provide crystal clear direction.
Digital training documents stress visualization and can support all types of media, including videos, flowcharts and more. This improves training comprehension by relying less on text or technical knowledge and more on the procedures themselves.

FDA Requirement
"Food processors must maintain a record of this training for each worker."
(Food CGMP Modernization, Training)
The Dozuki Solution: Reliable Training Records
In the eyes of the FDA, if a safety training wasn’t recorded, it didn’t happen. If employers are not recording and verifying that proper training has occurred, the employer is liable for the result.
Keeping records with paper training materials is cumbersome. It creates more work for teams, requires extensive reconciliation and takes up valuable time. Dozuki’s operator view allows you to record training procedures and collect training confirmation data simultaneously. This makes record-keeping an integrated part of the training process, rather than an additional task that requires follow-up from management.
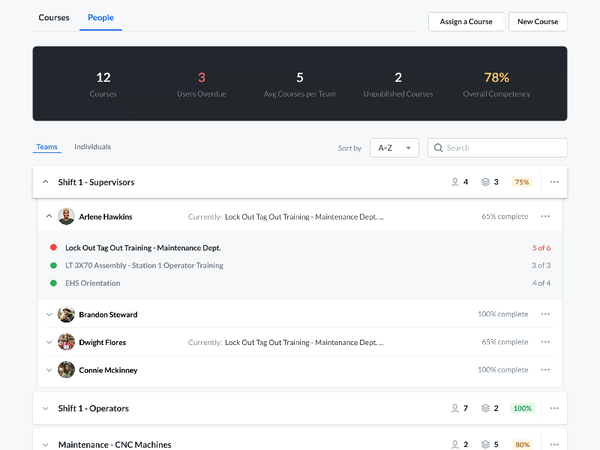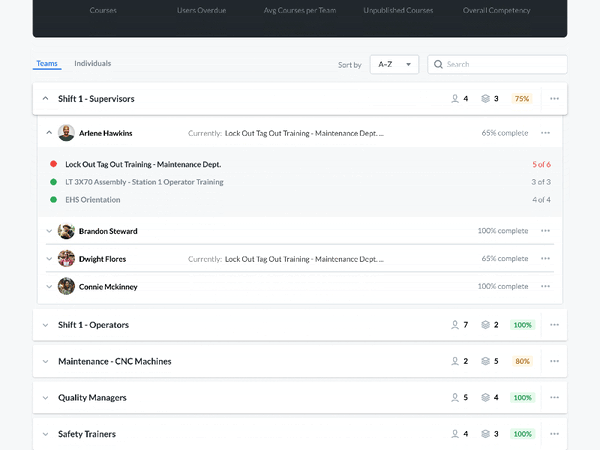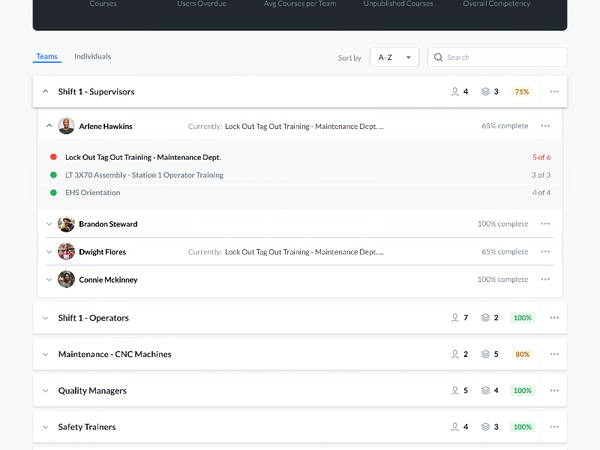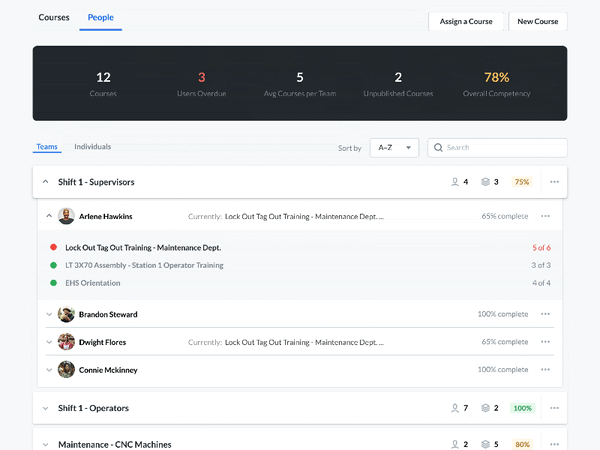
Modern Document Review
A critical part of the FSMA requires that any relevant sanitation procedures be accessible for an FDA official to review in a timely manner.
FDA Requirement
The Dozuki Solution: Auditor Collaboration
Communicate and work with your FDA auditors. You can receive feedback, answer questions, and review suggestions—without compromising document control. Required documents can be made available for review by anyone with the proper login credentials. This allows auditors to view the active sanitation procedures and how frequently they are being used—without having to make photocopies.
Setting up custom user groups and access permissions lets you limit document access to certain users. Built-in IP whitelisting makes it easy to authorize information access only within designated locations, preventing inappropriate use of company information.
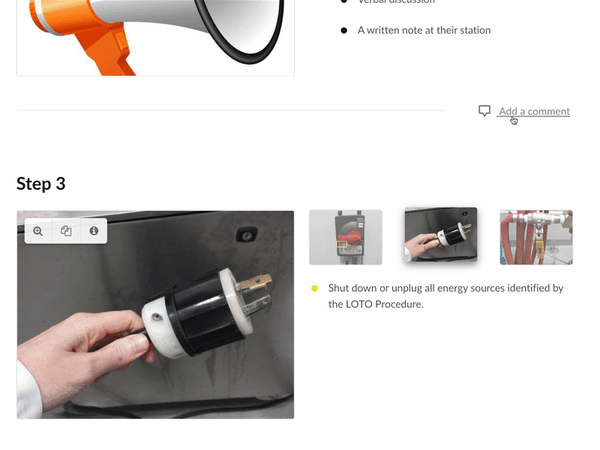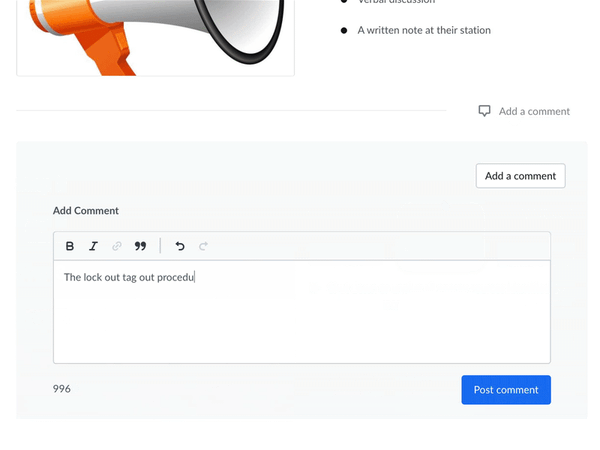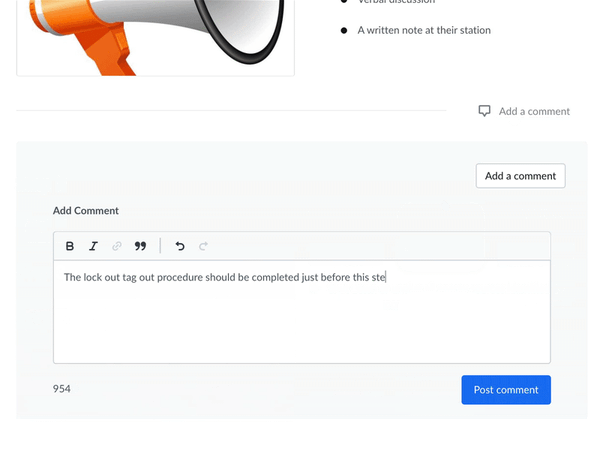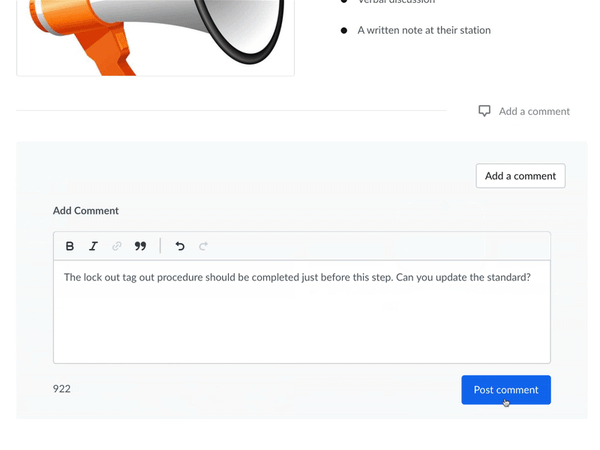
Scalable Corrective Actions
In a worse-case scenario, the FSMA gives the FDA power to implement mandatory recalls. Efficient approval processes and document release cycles affect how quickly corrective actions can take place. Corrective action requirements, are the equivalent of the FDA asking, “Hey, when you fix a problem, you need to tell us why, how, and when that happened.”
FDA Requirement
"Corrective actions must be documented with records."
(FSMA, Section 103 Hazard Analysis and Risk-Based Preventive Controls)
The Dozuki Solution: Streamlined Revision Process
When a corrective action is implemented, paper-based companies must revise all existing documents, store the previous versions, and make a record of when the revisions took place.
Custom approval processes in Dozuki allow you to instantly notify the right people when revisions need approval, making release cycles much faster. After documents have been approved, the updated version is instantly released and the previous version is archived for your records. This allows corrective actions to take place quickly and FDA auditors to have access to all the required records and confirmations.
Be Prepared for Future Audits
Paper-based documentation may work for now, but not for much longer. With the FDA requiring more and more robust documentation practices, digital documentation just makes sense.
Related Posts
View All Posts
Industry News
What The Doc Ordered: Better Performance in Medical Manufacturing
9 min read
The landscape of medical manufacturing has gotten more complex. Pandemic related shutdowns created global supply chain disruptions A batch of macroeconomic and regulatory...
Continue Reading
Product
How to Use Dozuki for Work Instructions
7 min read
Dozuki is the leading digital work instruction tool for industrial businesses all over the world. Our platform enables better employee performance by improving how you...
Continue Reading
Training
What to Look for in QMS Software
5 min read
Traditional quality management system (QMS) software does exactly what it’s supposed to do. Like file cabinets for the digital world, they organize and control your company...
Continue Reading